Sleeve Gastrectomy
Obesity is a serious, chronic disease that is known to reduce life span, increase disability and lead to many serious illnesses. These illnesses, including diabetes, heart disease, gallstones and stroke, are the co-morbidities of obesity. The Lewin study confirmed results from other studies in finding a direct correlation between increases in Body Mass Index (BMI) and increases in the prevalence of co-morbid conditions, especially type 2 diabetes, hypertension, heart disease, stroke and arthritis (Table: 1)
| Increased Risk of Obesity Related Co-Morbidities with Higher BMI | ||||
|---|---|---|---|---|
| Disease | BMI of 25 or less | BMI between 25 and 30 | BMI between 30 and 35 | BMI of 35 or more |
| Arthritis | 1.00 | 1.56 | 1.87 | 2.39 |
| Heart Disease | 1.00 | 1.39 | 1.86 | 1.97 |
| Diabetes (Type 2) | 1.00 | 2.42 | 3.35 | 6.16 |
| Gallstones | 1.00 | 1.97 | 3.30 | 5.48 |
| Hypertension | 1.00 | 1.92 | 2.82 | 3.77 |
| Stroke | 1.00 | 1.53 | 1.59 | 1.75 |
Table: Obesity Related Co-Morbidities with Higher BMI
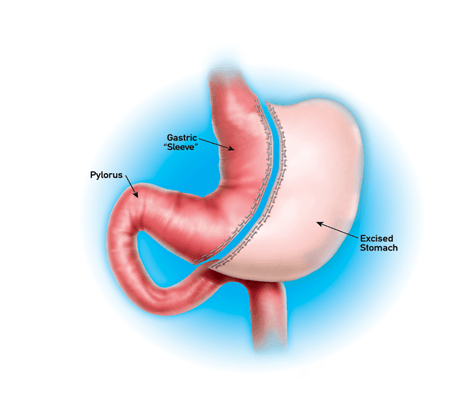
Figure 1: Laparoscopic Sleeve Gastrectomy
Laparoscopic sleeve gastrectomy is a restrictive procedure without the malabsorptive component present in other bariatric procedures (Figure 1). It involves resection of two-thirds of the stomach to provide increased satiety and decreased appetite. Through restriction in the stomach size, the intragastric volume is less able to accommodate a large volume of food leading to decreased food consumption. In addition, a lower volume of food leads to earlier distention of the stomach causing firing of stretch receptors in the gastric wall. These signals are communicated via the vagus nerve to the nucleus of the solitary tract in the brainstem which then signals to the hypothalamus and then the cerebral cortex leading to the perception of satiety.
Decreased levels of ghrelin that occur as a result of resection of the gastric fundus, the location of ghrelin production, also likely leads to greater satiety. Other purely restrictive procedures such as laparoscopic gastric band placement involve surgical implantable devices leaving a foreign body in place for future increased restriction if necessary, whereas the sleeve gastrectomy surgically and permanently reduces the size of the remnant stomach.
History of Sleeve Gastrectomy:
Sleeve gastrectomy for weight loss was first described by Marceau in 1993 as a component of biliopancreatic diversion. Super-morbidly obese patients have more complications after weight loss surgery and higher rates of failure. Therefore, a two-stage approach, consisting of Laparoscopic sleeve gastrectomy followed by laparoscopic Roux-en-y gastric bypass (LRYGB) was first performed by Regan et al. in 2003 to overcome these challenges in the super-morbidly obese patients. Laparoscopic sleeve gastrectomy revised to laparoscopic BPD-DS has also been described to achieve similar results. Over time the operative approach to morbid obesity through utilization of Laparoscopic sleeve gastrectomy has been modified and it is often now used in isolation due to its demonstrated effectiveness in regards to EWL and resolution of obesity comorbid conditions. Laparoscopic sleeve gastrectomy has also been utilized in a variety of other settings creating much interest into this procedure.
Operative Technique of Sleeve Gastrectomy:
Antiembolic precauations are taken and appropriate preop antibiotics are administered. A 12-mm optical trocar is placed under direct vision approximately 15 cm below the xiphoid and 3 cm to the left of midline (Figure: 2).
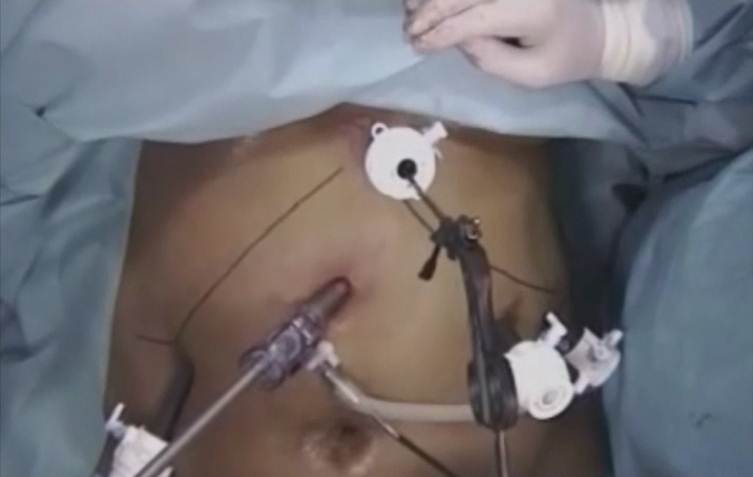
Figure: 2 Port position in sleeve gastrectomy
A 45-degree angled laparoscope is placed through the port into the peritoneal cavity and 12-mm port is placed in the left lateral flank, medial to the edge of the colon with the patient in a supine position and at the same level as the periumbilical port. Next, a 5-mm trocar port is placed along the left subcostal margin between the xiphoid process and the left flank port. Another 12-mm port is placed in the right epigastric region and a fourth 12 mm port was placed in the mid-epigastric region caudal and medial to the previous port. The liver is elevated and this provides adequate visualization of the entire stomach during the gastrectomy.
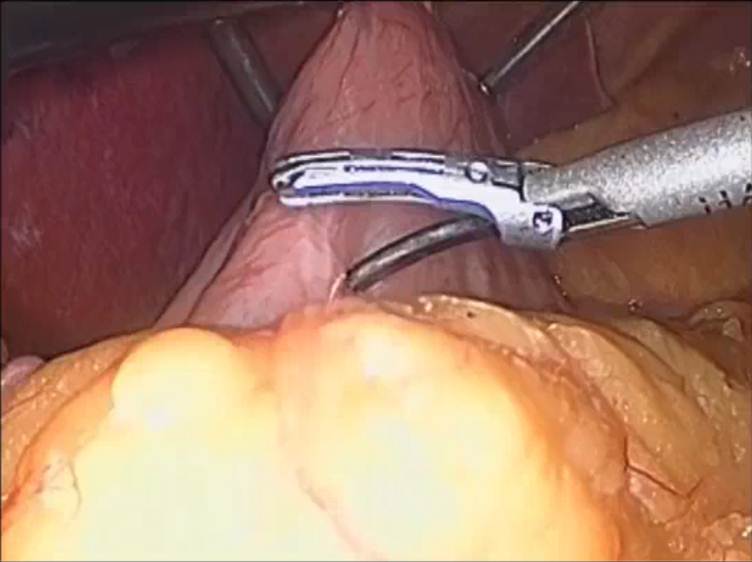
Figure 3: pylorus of the stomach is identified
The pylorus of the stomach is then identified and the greater curve of the stomach elevated (Figure 3). A ultrasonic scalpel is then used to enter the greater sac via division of the greater omentum. The greater curvature of the stomach is then dissected free from the omentum and the short gastric blood vessels using the laparoscopic ultrasonic scalpel.
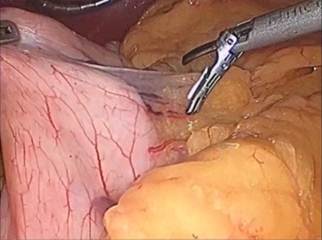
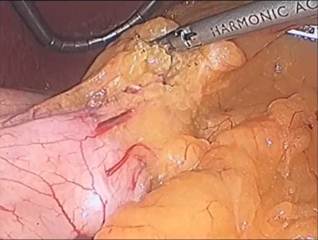
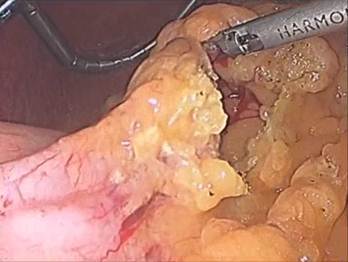
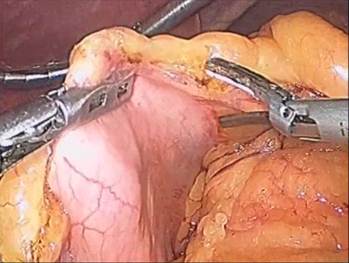
Figure: 4 A,B,C,D
The dissection is started 5 cm from the pylorus and proceeds to the Angle of His (Figure 4 A – D). A 9.8 mm gastroscope is then passed under direct vision through the esophagus, stomach, and into the first portion of the duodenum. The gastroscope is aligned along the lesser curvature of the stomach and used as a template to perform the vertical sleeve gastrectomy beginning 2 cm proximal to the pylorus and extending to the Angle of His.
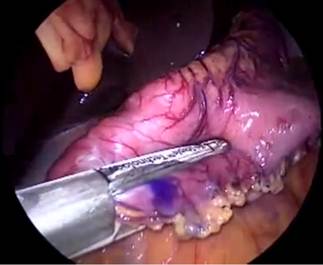
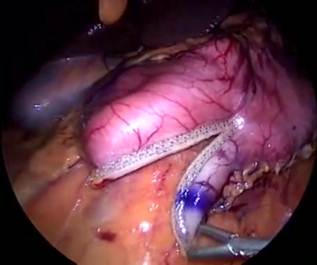
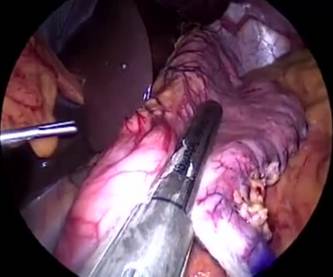
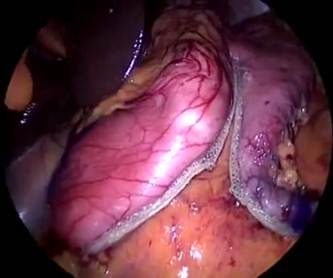
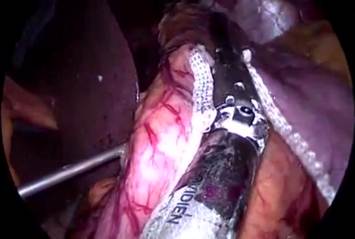
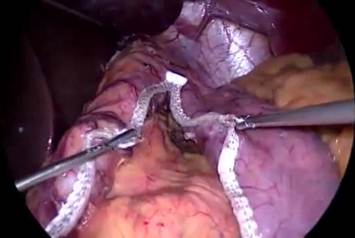
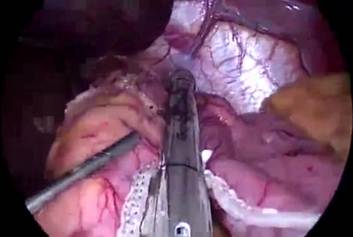
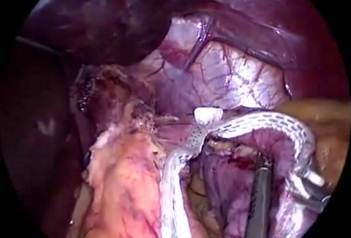
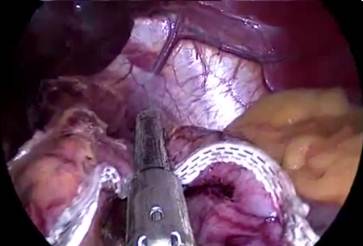
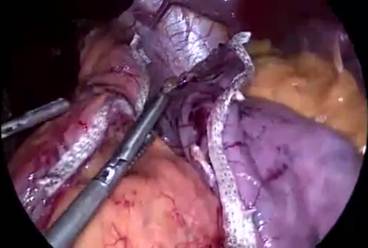
Figure 5: A to J showing steps of sleeve gastrectomy
An endoscopic linear cutting stapler is used to serially staple and transect the stomach staying just to the left and lateral to the endoscope. The gastrectomy is visualized with the endoscope during the procedure. The transected stomach, which includes the greater curvature, is completely freed and removed from the peritoneum through the left flank port incision (Figure 5: A TO J) . The staple line along the remaining tubularized stomach is then tested for any leak through insufflations with the gastroscope while the remnant stomach is submerged under irrigation fluid. The staple line is concurrently evaluated for bleeding both intraperitoneally with the laparoscope as well as intraluminally with the gastroscope. A 19-French Blake drain is left in the left upper quadrant along the sleeve gastrectomy staple line. We do close the fascia of the left flank port site with an absorbable suture on a transabdominal suture passer, to prevent bowel herniation but do not close the fascia defects at the remaining port sites.
Technical Variations of Sleeve Gastrectomy:
Several technical variations around the laparoscopic sleeve gastrectomy happen to be reported. The main difference is the size dilator utilized to create the sleeve during stapling and transection. A wide range sizes for bougie dilators happen to be employed to size the gastric pouch. Different bougie sizes utilized by a single group failed to demonstrate any effect of bougie size, indicating bougie size might have little impact on achieved weight reduction. Some research has employed the use of a 9.8 mm gastroscope for gastric sleeve sizing which falls across the smaller range of sizes employed for gastric sleeve sizing. It provides the advantage of allowing intraluminal visualization during gastric stapling, and post-stapling visualization for evaluation of staple line hemorrhage as well as for a post-transection leak test. Whether or not the small diameter leads to a heightened risk of stricture is not known.
Post-operative Management
On post-operative day one all patients undergo a gastrograffin swallow study to evaluate for leak or stricture. We do find that some patients experience delayed passage from the contrast likely due to edema within the narrow gastric remnant. Delayed passage of contrast does not prevent discharge in the hospital, however, as it is felt this is due to post-operative edema and can resolve over the next a few days post discharge. Following gastrograffin swallow, if the patient is medically stable with adequate pain control, the patient is discharge after dietary instruction with a bariatric dietician to follow-up 7-10 days post discharge. Subsequent follow-up occurs at 30 days, 3 months, Six months and 1 year post surgery after which yearly thereafter. Routine bariatric labs to evaluate for nutrient deficiencies are carried out at Six months, twelve months after which yearly following sleeve gastrectomy.
Outcomes Following Laparoscopic Sleeve Gastrectomy:
Initially LSG was utilized like a component of a two-staged surgical approach to morbid obesity, followed by LRYGB. When utilized because the initial stage of the two-staged approach, patients experienced decreased weight, comorbidities indicates LSG may alone reduce the potential morbidity and mortality in high-risk patients just before LRYGB. Resolution of comorbid conditions including hypertension, diabetes, obstructive sleep apnea, hyperlipidemia, and gastroesophageal reflux disease have been reported with continued improvement after revision. Cottam, et al. reported a significant decrease in comorbid conditions from 9 conditions present just before LSG, to six conditions present after LSG and prior to LRYGB, with a further decrease to 2 conditions after LRYGB. Resolution rates include hypertension, 15-100%, diabetes, 47-100%, obstructive sleep apnea, 56-100%, hyperlipidemia, 45-73%, and gastroesophageal reflux disease, 70-80%. Although variable, these results are promising and further long- term follow-up with randomized controlled trials comparing other weight loss procedures is essential.
Complications of Sleeve Gastrectomy:
The incidence of complications after Laparoscopic sleeve gastrectomy continues to be reported at 0-24%. Several large series have demonstrated probably the most frequently encountered major complications included leaks (0-10%), suture line hemorrhage (0-10%), and major organ injury (0-5%). Rates of post-operative stricture are reported at less than 1%. The general mortality rate is estimated at 0.39% within the literature.
Laparoscopic sleeve gastrectomy vs. Laparoscopic Gastric Bypass:
There is much interest into the risk/benefit profile of Laparoscopic sleeve gastrectomy versus LRYGB due to the perceived increased risks associated with LRYGB. Primary Laparoscopic sleeve gastrectomy has been recently when compared with primary LRYGB inside a group of Indian patients. At twelve months follow-up reduction in excess weight loss was 76% within the LSG group and 62% in the LRYGB group. Comorobidity resolution in LSG/LRYGB were 83%/92% resolved for hypertension, 78%/98% resolved for diabetes mellitus, 75%/78% resolved for dyslipidemia, and 91%/100% resolved for gastroesophageal reflux disease. Only one major and one minor complication took place each group. A prospective, randomized-controlled trial involving 16 patients in each group demonstrated an extremely increased reduction in excess weight loss in LSG as compared to LRYGB at 6 months and something year post-operatively. However, a recent prospective randomized controlled trial has reported early Three month results demonstrating equivalency between LSG and LRYGB in regards to reduction in excess weight loss and resolution of diabetes. It's yet unclear which procedure achieves greater comorbidity resolution and reduction in excess weight loss as comparative studies still have short-term follow-up. LRYGB has been demonstrated to have a reduction in excess weight loss of 63% inside a large meta-analysis, although several large studies demonstrate EWL between 68-77%. Although variable answers are achieved between surgeons and centers, this estimate falls in the selection of that seen with LSG, although at the upper end from the reported range of EWL after LSG. A lot of information is not yet readily available for LSG making comparison to LRYGB difficult. Additionally, resolution of comorbidites and complication rates are difficult to check between your two techniques. Two of the major complications after LRYGB include anastomotic leak (2-4%) and gastrojejunal stricture (0.5-4.9%). Mortality rates are lower in both LSG and LRYGB. Comorbidity resolution is nice in both techniques however the literature isn't clear on which technique is superior in relieving the most popular obesity comorbid conditions. Just one benefit of LSG when compared with malabsorptive procedures for example LRYGB and laparoscopic BPD-DS is the fact that endoscopic procedures difficult after surgical reconstruction from the small bowel are possible after LSG. This becomes particularly important in evaluating patients with suspected gastritis or biliary pathology which become hard to evaluate endoscopically after LRYGB.





